Collagen, one of the major components found in skin, serves an important function in leather – to provide mechanical support by withstanding loads acting on the material. Here we discuss the basis of the mechanical stability of collagen from macro to nano scale that underpins the functional significance of collagen.
There are several types of collagen but here we focus on those that participate in higher-order assemblies such as networks, filaments, microfibrils, fibrils and fibres/fascicles. These assemblies collectively form a hierarchical architecture in the tissue from the molecular to the macroscopic level. The functional significance of collagen is a subject of ongoing research, as the knowledge gained can direct the development of new technology like leather design and production.
Biomechanical engineers regard soft connective tissues such as tendons, ligaments and skin as biological examples of fibre-reinforced composites comprising collagen fibrous structures embedded in a hydrated proteoglycan-rich extracellular matrix (ECM). With a remarkable high-tensile stiffness and strength, these collagen fibrous structures are responsible for withstanding external loads that act on the tissue. From a fibre composite perspective, while the mechanical properties of the tissue are attributed to collagen, it is important to emphasise that the interfibrillar matrix facilitates the load transfer from the hydrated PG-rich matrix (the weak phase) to collagen (the strong phase); minimises direct contact between fibres by ensuring that the individually fibres are separated, which can in turn prevent a brittle crack from passing completely across a section of the composite; and protects the surface of the individual fibres, otherwise the fibre surface may experience abrasion by direct sliding contact and this could compromise the mechanical properties.
Animal hides and skins are tough and strong materials. Transforming them into a variety of useful and desirable products involves a chain of processes. Several processes involve subjecting the collagen in the materials to chemical and mechanical modifications – to treat and soften the hides – while minimising possible damage to the properties of toughness and strength of the hide. There is also a need to design efficient methods that are environmentally sustainable for processing leather. At the tannery, a significant amount of water and chemicals are often used, but the carbon footprint is further enlarged as energy is also required to drive these chemical reactions.
A collagen molecule is composed of three polypeptide chains exhibiting a triple-helical structure, and the molecule is often referred to as tropocollagen molecule. How the tripeptides contribute to the stability of the collagen molecule has been a subject of great interest for decades.
Factors affecting collagen stability
With regard to mechanical stability, the subjects of discussion are leather processing, and agents of deterioration, namely heat and mechanical wear. Regarding heat, the discussion on deterioration effects is complemented by highlights on some recent findings on tanning process as a protection against heat. Regarding mechanical wear, the discussion is on structural changes and corresponding mechanical changes when leather is in service, complemented by differentiating these effects from mechanical treatment to leather during processing. Obviously, deterioration due to heat and mechanical wear are two of the many factors such as oxidation, metals and salts, and water.
Collagen makes up about 70–80% of the dry weight of the skin. So how different is leather collagen structure from skin? The processing of skin leading to leather removes many ECM components, like epidermal cells, proteoglycans, elastin, but collagen appears not to be dramatically affected by the processing, even after liming, bating and pickling are applied. The collagen fibrils now become connected by synthetic chemical bonds as well as natural chemical bonds. These bonds may enhance the yield strength of the leather because the fibrils may be unable to pass one another easily.
However, these bonds may also undesirably stiffen the material, so glycerol is introduced into the interfibrillar matrix during fat liquoring. The final product leads to two distinct layers in the leather, which are vaguely related to the dermis of skin: a fine densely packed fibrous layer and coarser layer we refer to as the grain and corium layers, respectively. Much of the organisation of collagen found in skin is retained in these layers. The other features of collagen fibrous structures in the skin – namely high slenderness and characteristic light-dark bands referred to as the D periods on the structure – are also retained in leather.
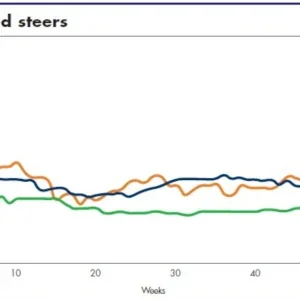
The general mechanical response of connective tissue when subjected to an external load follows a stress-strain profile, and there are eight main stages of processing leather from skin, namely fresh green, salted, pickled, pre-tanned, wet-blue, retanned, dry crust and dry crust-staked. How do the stressstrain profiles look like for leather during the different stages of processing? It turns out that the stress-strain curve varies dramatically with the respective stages.
It is easy to point out the similarities in regard to the features; namely, the existence of a toe region, non-linear (attributing to elasticity-related mechanisms) region in the low-stress region; point of inflexion (attributing to failure mechanisms, such as yielding, leading to plasticity) at higher stress region; and abrupt reduction in stress as the material breaks apart, beyond the maximum stress point. These features are reflected in skin as well as ligaments and tendons. The more interesting observations are the effects from the various processing stages as reflected in the extents of the stress and strain, as well as the stiffness at low-stress regions. The different effects arising from the variety of processes underpin the collagen mechanical stability at different hierarchical levels.
Unfortunately, current findings of how the stress strain behaviour leather changes during processing, by attributing to the collagen fibril alignment (fibrillar level) and collagen D-spacing (molecular level), do not lend themselves to a complete understanding, as the effects at the other hierarchical levels in leather are still not clear. These are important considerations for further study, especially where newer findings, such as new fat-liquoring agents, and recommendations to lower the amount of chromium use in stabilising collagen, have been recently proposed.
Tanning is intended to stabilise the collagen molecule by creating a more permanent bonding between the helices. The degree of stability depends on the tannin molecule, with condensed tannin type being more superior than hydrolysed tannin type; the former yields lower mass loss (TGA curves) and smaller rate of mass loss (DSC curves) over a range of high temperatures. Currently, findings of what really happened during heating by attributing to collagen molecular level effects – for example, mechanics of bonding – do not lend themselves to a complete understanding as the effects at the other hierarchical levels in leather are still not clear.
Mechanical wear and tear
How mechanical loading affects the mechanical stability of collagenous material such as leather has been dealt with in studies on leather processing and leather during service. Repeated loading that forms part of leather processing, namely milling and staking, is intended to yield the desired softness in leather.
Milling is likened to a preconditioning process to ensure consistency of results, borrowing from biomechanical testing of soft tissue, prior to the leather being deployed for use. At the macroscopic level, analysis of the hysteresis curve revealed that a milled leather results in smaller energy loss than unmilled leather. As the number of cycles increases, the strength increases but the strain at fracture decreases. Clearly there is an optimal level to achieve high strength without compromising too much on reduction in the extensibility. At the fibrillar level, milling ensures that collagen fibrils are recruited into the desired orientation. At the tropocollagen molecular level, the cyclic stress experiences by a dry leather may minimise hydrogen bonding within the fibrils. Static stretching of leather material, along its long axis (parallel to the backbone), to a desired length and maintaining the leather in this state over time has been proposed as part of a leatherprocessing stage (namely during the wet-blue stage) intended to achieve maximal area, which is important for optimising the profit, with consequential enhancement to collagen mechanical stability. At the macroscopic level, stretched leather stiffness is dramatically higher (particularly those treated to low angles with respect to the long axis) than those without pre-strain; the stiffness increase also depends on the amount of stretch applied during the pre-strain treatment. At the fibrillar level, this is explained by the result of fibrils oriented predominantly along the pre-strain axis.
Tearing could occur when the leather is in service. In the latest attempts to understand the mechanical stability of collagenous materials, like leather, to resist tearing, researchers found that there was an appreciable difference in the tear strengths (whether torn in parallel or perpendicular to the backbone) in leather (tanned) processed without strain and leather prepared by tanning under strain; the tear strength increases in both directions. This finding is important because it correlates to orientation changes at the fibrillar level, whereby a greater degree of alignment was observed with pre-strained leather compared with unstretched leather. Of note, it has been pointed out that tannins may help to increase wear resistance. This is because hydrolysis of tannins, which can occur within the leather and is not desirable from the point of view as a loss of tanning material, results in carboxylic acid moieties by-products, which is deposited in the tanning pits, and this may contribute to the water resistance and wear properties of the leather.
Collagen fibril network
The network of collagen fibrils in dermal tissue features a somewhat randomly aligned state. However, when the tissue is deformed under a tensile load, the entire network can be recruited in tension by realigning the fibrils through a significantly large angular displacement – 50°, for example – in the direction of the applied load. Collagen is mechanically stabilised in this way for as long as the load does not exceed the fracture strength. Since the degree of fibril orientation determines the tear strength of the leather, high tensile strength may be achieved for the finished product, which possessed highly aligned fibrils (if the strength is measured in the stretched direction of the load).
The tensile strength depends on the direction of loading on the leather material in leather with highly aligned fibrils. The tensile strength, as well as stiffness, decreases as the angle of loading (with respect to the backbone) increases. However, finished products of leather with highly aligned fibrils may not be desirable, especially if looseness occurs, a defect that can be detected during quality control and decreases the product value because it does not make the leather look good. Some parts of a hide, namely shoulders and flanks, can give rise to looseness. The highly aligned fibrils in loose leather occur throughout the thickness of leather, compared to tight leather; loose leather is also found to have less densely packed fibrils, particularly in the lowergrain region.
While the tear strength in the direction parallel to the aligned fibrils is high, the tear strength in the direction perpendicular to the aligned fibrils is low. It is found that the looseness manifests during leather processing and exacerbates at different stages. In particular, the fibril alignment is shown to develop during the wet-blue stage; the degree of fibril alignment follows a trending increase as the material undergoes different stages of processing. Unfortunately, what exactly causes looseness still isn’t clear.
Several factors can affect the alignment of collagen fibrils, namely hydration, leather thickness and prestrain treatment. The fibrils in dehydrated leather materials are less aligned than hydrated ones. Leather thickness also affects the fibril alignment in that the fibril orientation in the grain layer is vastly different from that in the corium layer. The amount of pre-strain influences the stiffness; stiffness increases with increasing pre-strain values dramatically. It should be emphasised that changes in fibril alignment is not merely a 2D planar effect; a proportion of the realignment of the fibrils also comes from the planes perpendicular to the surface of the leather during pre-strain and it is proposed that this proportion could also contribute to the increased stiffness.
Fibril-matrix interface
When collagenous tissues deform under an external load, the deforming hydrated PG-rich ground substance shears on the collagen fibrils; the natural cross links between fibrils and between fibrilmatrix are deformed, and this generates an interfacial shear stress. Consequently, shear on the fibril causes the fibril to stretch and generate stresses to resist the external load that is attempting to pull the tissue apart.
During leather processing, various components of the hydrated proteogylcan-rich ground substance in ECM are removed, and the intermediate product at the pickled stage would have fewer natural cross links. However, new cross links are created during the pretanning and wet-blue stages. It was observed that the D period of collagen during the pickled stage was much higher than that during the wet-blue stage, suggesting that the collagen fibrils were appreciably stretched in the former as compared to the latter. This could compromise the collagen stability as shown by the small strain range in the former as compared to the latter. Nevertheless, the presence of new cross links in latter stages up to the finished product suggest that these shear stress responses are expected to be applicable to the fibril-matrix interface in leather material.
With respect to native tissue, the absence of GAGs (removed by Chondroitinase ABC) showed no appreciable change to the orientation index. GAGs have been the ‘usual suspect’ for natural cross links for quite a long time. These natural cross links at the fibril-matrix interface facilitate the stress transfer within ECM, based on observation of micrographs of GAG side chain, associated with proteoglycans (PGs) bound on collagen fibrils. However, in the past 10 years or so, investigations to study how tissue mechanical properties are compromised by removing GAGs (by Chondroitinase ABC) have yielded negative results. Although the exact nature of the natural cross links are not yet known, introducing artificial cross links (using glutaraldehyde) into the collagenous tissue can result in a significant effect on the orientation index when the tissues are stretched, implicating that the artificial cross links actually work to anchor between fibrils, and to realign the fibrils in the direction of the applied load. Glutaraldehyde is a tanning agent that functions as a collagen crosslinker, intermolecularly and intramolecularly, forming covalent bonds for interconnecting the collagen fibrils, as well as polymerisation of glutaraldehyde to increase the network density.
Alternatively, it has been proposed that collagen fibrils may interact directly without the help of extrafibrillar molecules by attributing the interaction to fibril branching. During development, collagen fibrils grow in diameter and in length through both end-toend and lateral fusion, resulting in fibril branching; thus fibril branching is also regarded to facilitate interfibrillar load transfer between the small and large-diameter fibrils. Given that most tendons exhibit a distribution of small and large fibril diameters, small-diameter fibrils may play an important function to connect and transmit force between the larger load-bearing fibrils in tendon.
At the molecular level, the mechanical stability of the fibrous structure may be better understood from studies of the assembly of the tropocollagen molecules into fibrils, which is regarded as a thermodynamically (entropy)-driven process under ordinary/physiological conditions. Tropocollagen molecules resemble a triple helical arrangement of three coiled collagen-protein chains, linked together by hydrogen bonds. When the molecule is stretched, at low displacement, the force generated in the molecule is low as the helix becomes uncoiled, but as the displacement increases, the force increases. These mechanical characteristics and structural bonding provide the cornerstone for understanding the ability of fibrous structures such as collagen fibrils to take up stress when stretched.
The overall mechanical response of the molecule may be described by a worm-like chain model. This force-displacement profile may be divided into three regimes; the low (near-constant) force regime is known as the entropic elastic regime; the linear regimes comprised of a low stiffness regime where the tropocollagen molecule continues to uncoil; and a high-stiffness regime where the molecule is fully stretched over the ‘backbone’ so that further stretching will result in rupture.
The mechanical stability of the molecule is parameterised by the molecular contour length and the persistence length of the molecule at a predetermined absolute temperature. How the changes at the molecular level – namely the characteristic banding pattern, or D period, in collagen – contribute to bulk level behaviour such as dehydration, tanning and stretching, have been reported in several studies. You may need no reminding that in native tissues tropocollagen molecules in fibrils are staggered axially, resulting in a periodic light-dark bands with a D period of about 67nm when viewed under an electron microscope. The light bands are associated with gaps (region of low-density collagen packing) between the ends of two molecules, while the dark bands arise from molecular overlaps. The nature of the D period has been well-explored for a long time using data from X-ray diffraction peak patterns of hides.
Other tanning agents such as fat liquor can penetrate into the fibril and interact with the molecules to change the D period. Overall, the D period is shown to decrease with progressive leather processing stages. With regard to dehydration studies, it is well known that the D period decreases on drying. The reduced D period reflects the overall reduction in the characteristic gap (where collagen packing density is low) and overlap regions, possibly associated with deformation of the collagen crystal structure. Upon rehydration, swelling of the fibrils occurs but a critical point is reached beyond which the fibril volume remains constant.
Thereafter, only the interfibrillar matrix continues to swell. In some species, the D period is also dependent on the location in the leather (throughthickness), such as the corium and grain layers. However, the extent of the differences with respect to the location may be species-dependent. The D period increases with increase in the tensile strain of leather as the tropocollagen molecules elongate, and slip with respect to adjoining molecules, along the fibril axis, which changes the length of the gapoverlap regions. It is important to emphasise that large-scale changes in a collagenous material, such as leather stretching from a relaxed state or past the point of yielding, cannot be properly understood in terms of what a single tropocollagen molecule is doing using the WLC model, although the WLC is a useful model for understanding how a molecule responds to an external load.
Current interest in multi-scale modelling of the collective ‘many-molecule’ behaviour (for example, by incorporating the WLC model) at the molecular level, the collective ‘many-fibril’ behaviour (by incorporating stress transfer mechanisms) at the fibrillar level, and the correlations that must be established between the different levels across the full length scale as reported in several fundamental papers, may be the answer to understanding the stability of collagen in leather. To address this approach would require establishing a conceptual framework underpinning organised information of the structure-function relationship of collagen.
Hierarchical architecture of collagen
In 2014, a strategy was proposed to help advance our understanding of the structure-function relationship of ECM. This strategy underpins a concept of organised information addressing a framework for the mechanisms of stress uptake in the structural units reinforcing the tissue at the respective levels of the hierarchical architecture. The framework that I have constructed takes the form of a table initially, but this eventually led to a schematic of structural levels versus loading stages, regulated by known mechanisms at each structural level and corresponding loading stage.
The framework was aimed at facilitating comparison of individual stress uptake mechanisms between different tissue structural components of the same structural levels and across different structural levels, comparison of mechanical pathways, and prediction of new interconnection between existing mechanisms.
The framework for describing the hierarchical architecture can be applied to leather by organising findings from experiments and predictions from analytical/computational models in leather studies. The findings would serve to aid researchers’ understanding of the structurefunction phenomena or to inform manufacturing decisions with socio-economic consequences. While many studies on collagen fundamentals have used computational biomechanics models, there is a dearth of reports from analytical/computational modelling in leather studies. It is likely that increased computer speed and better specialist software will enable collagen modelling – for exapmle, in silico leather – to be carried out, to be applied to an increasingly wide range of problems, and to be deployed in the manufacturing of leather where modelling could be incorporated as part of the process. On this note, it is important to deal with model credibility where computational biomechanics models would be used for leather property predictions.